Client Services
Our clients include companies in the Chemicals Industry both in Australia and overseas
Since commencing business in 1990, we have had hundreds of clients from all Australian states and in several other countries including UK, USA, Italy, Israel, New Zealand, Malaysia, Brazil, China and Canada. These companies range in size from one person companies with a few good ideas that need developing, through to the largest of trans-national companies with turnovers of many billions of dollars per year and staff of many thousands.
These companies come along to us because we reliably offer the best value-for-money speciality service available. We have come by much of our business by personal recommendation. This is the best advertisement for any company; that existing clients recommend our services to others.
Specialty services include (see Section links below):
- Regulatory Affairs Consultancy (registration of Agricultural and Domestic Pesticides and Veterinary Products with Australian Government Authorities) in Canberra.
- Safety Data Sheets (SDS) prepared to Australian and New Zealand format
- Labelling requirements and wording service
- General Chemical Consulting, Research and Investigatory Services
These services are offered after a thorough discussion with clients. Likely costs and timings are discussed, and a solution is offered. If agreed, the project may then proceed.
In order to properly do our job, we often need to be provided with detailed formulation details. If necessary, we are happy to sign secrecy agreements and have done so with many companies. Our integrity in such matters is assured. In the time we have been in business we have never compromised the trust our clients have placed in us.
Regulatory Affairs and Labelling
To ensure the continued high standard of Australian primary produce in the world marketplace, Australia places high emphasis on pest and disease free produce which is achieved through the correct use of high quality agricultural chemicals while maintaining safety through the minimisation of chemical residues in treated produce.
In Australia, the manufacture and sale of Agricultural and Veterinary Chemicals are regulated by the Australian Pesticides and Veterinary Medicines Authority (APVMA). To obtain registration a product must have been shown to be safe and effective when used as directed. The manufacture and use of the product should also avoid harmful effects to workers and the environment.
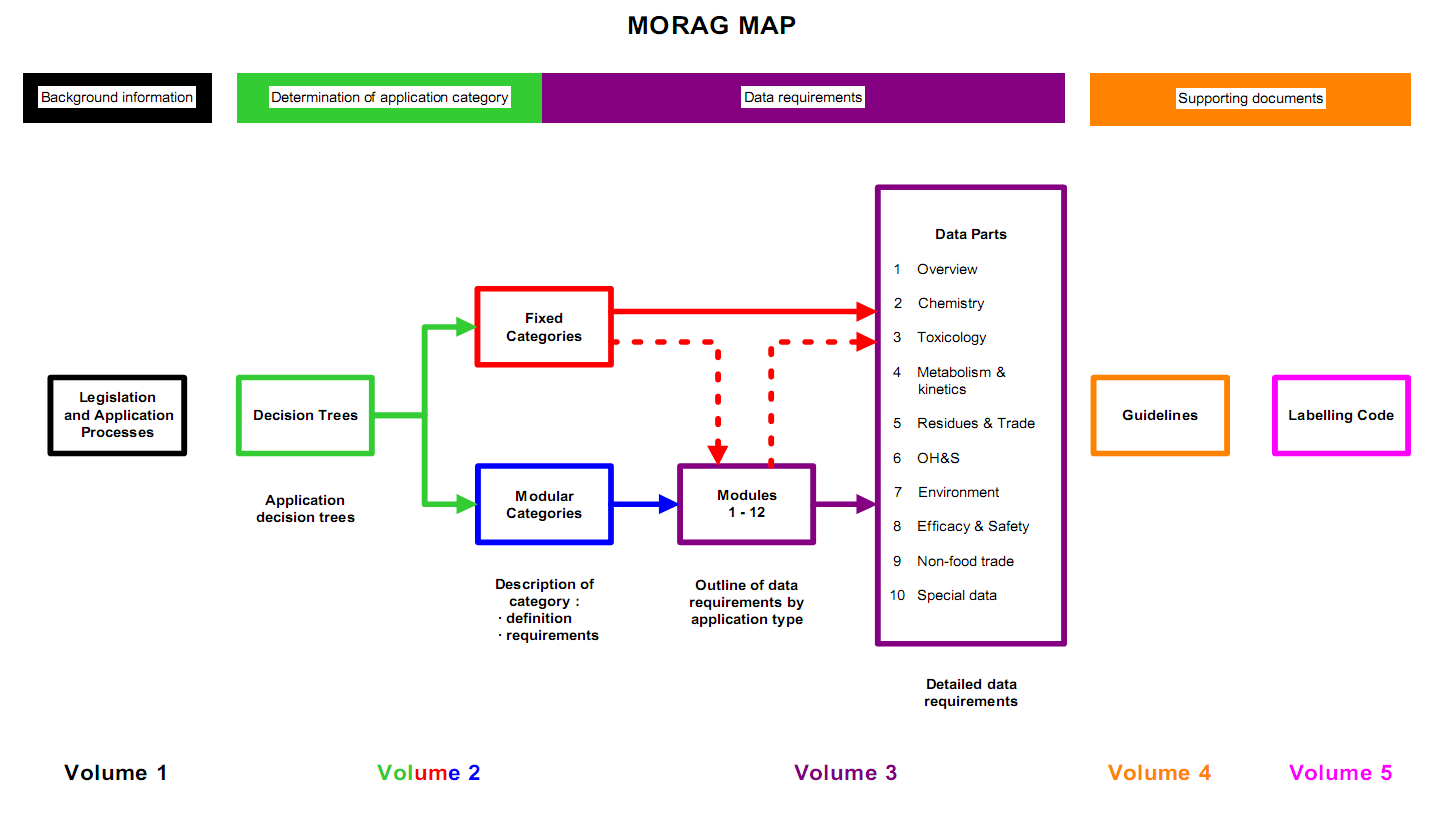
During the application for registration, the product label and supporting documentation which covers chemistry, manufacture efficacy and safety must be evaluated and approved by the APVMA. The registration of a product should then be maintained by updating labels to comply with changing guidelines and supplying data to the APVMA on a regular basis as required. The MORAG (Manual of Requirements and Guidelines) schematic illustrates the Australian Pesticides and Veterinary Medicines Authority requirements and guidelines for applications to register or approve agricultural chemical products, labels, active constituents and permits.
Kilford & Kilford Pty Ltd offers a Regulatory Affairs service which covers all aspects of approval of active constituents and registration of end-use products, including, feasibility studies, coordination of testing programs, preparation of documentation, drafting of labels, liaison with regulatory authorities, and checking of artwork. Throughout a project we regularly report progress to our clients and are constantly aware of marketing schedules. As an additional service to our clients we are able to maintain ongoing records for registered products to ensure their continued compliance with the latest requirements while maintaining a watch for policy changes which may affect the future of products.
For overseas clients without an office in Australia we can act as their agent until product registrations are finalised, since applicants or their agents must be Australian residents.
Safety Data Sheets (SDS)
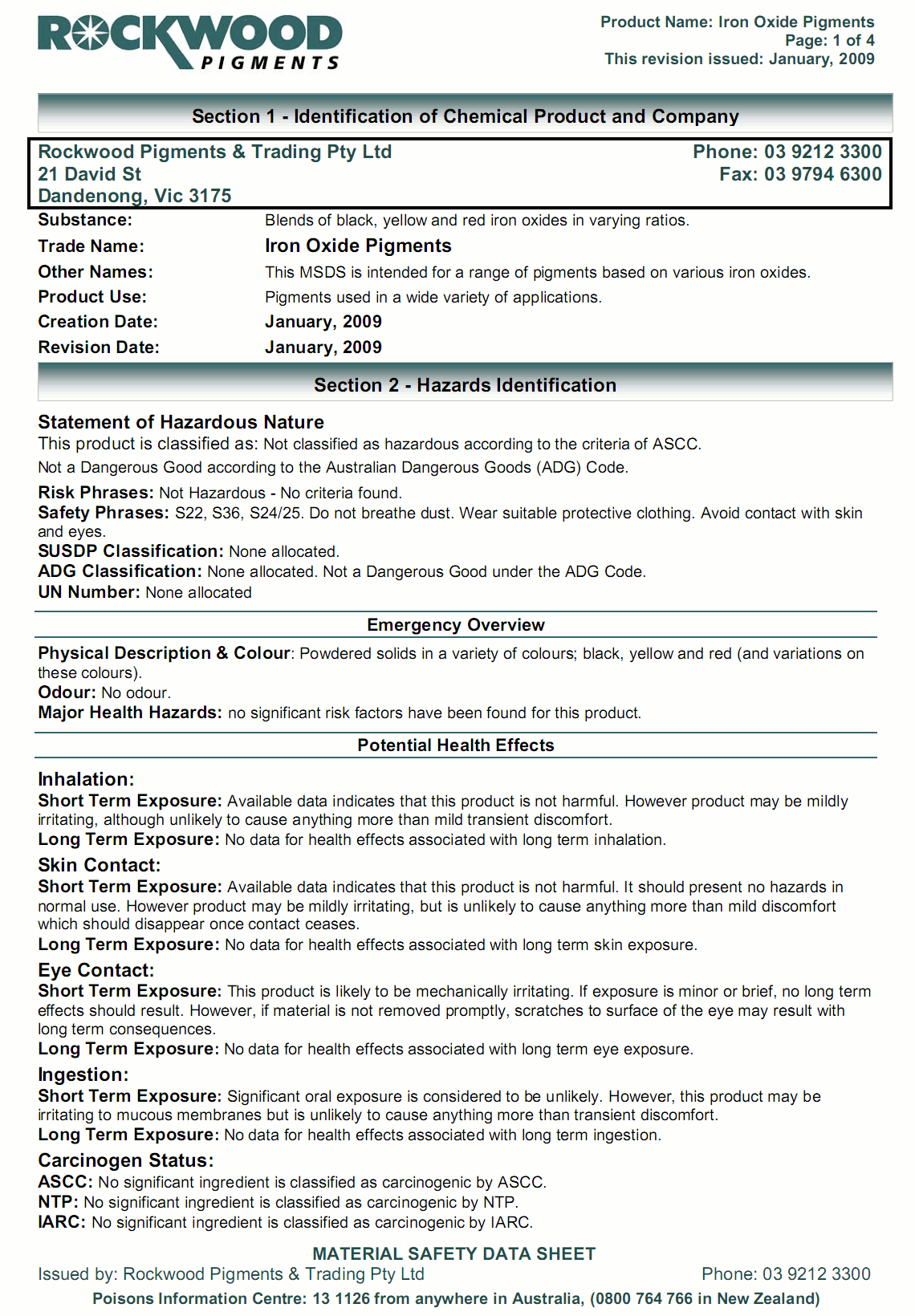
The format for Australian SDSs and their content is established by Safe Work Australia and can be downloaded at Model Code of Practice - Preparation of Safety Data Sheets for Hazardous Chemicals. This 16 point format is similar to the format that has been used in Europe, USA and other parts of the world for many years. In fact, most of our work has been done in that format (or similar to it) since early in 2002 and all since early 2009.
Under various current or proposed state regulations (which are very similar, being based on model regulations published by ASCC), employees shall have ready access to SDSs for hazardous substances at their workplace. They should also have access to the Code of Practice and receive instruction on interpreting the content of SDSs. A sample of a properly formatted SDS is provided in the Code of Practice. All SDSs issued in Australia should provide a contact point in Australia for further information on the product.
Other events which may trigger the review and re-issue of SDSs include changes in the formulation which:
- affect the hazardous properties of the substance
- alter the form or appearance of the substance
- alter the mode of application of the substance
- whenever there is a change to the substance which alters the health and/or safety hazard or risk
- whenever there is any new health and/or safety information on the substance
- to reflect new regulations and standards
In any event, no SDS should be issued which has not been reviewed and reissued in the past 5 years.
Kilford & Kilford Pty Ltd can prepare your SDSs in Australian format. This is almost identical with the New Zealand format and the two are virtually indistinguishable. The completed document may be supplied in hard copy, on disk or appended to an email as a pdf.
As part of the service we determine, where applicable, UN numbers and other details such as:
- Hazchem Code
- Packaging Group
- Correct Shipping Name
- Poison Schedule
- Risk and Safety Phrases
- Australian Exposure Standards
- Presence or absence of ingredients on the Public Core Inventory (AICS)
To determine this, we need formulation details and as much further information on products as possible. We do not have laboratory facilities and therefore cannot measure physical properties. We have many well known clients. If needs be, we are happy to sign any reasonable secrecy agreement. In addition, we would be happy to refer bona fide enquirers to some of our clients by way of referees.
Our SDSs and labels are written with the aid of a complex and smart series of macros and client templates using Visual Basic for Applications, the Macro language for Word in Office 2003. The program code runs to more than 15000 lines. This system incorporates standard risk and safety phrases, and cut-off points from the List of Designated Hazardous Substances. In addition, UN numbers and associated Hazchem codes, packaging groups and Proper Shipping Names come from the latest Australian Dangerous Goods code, and is kept as an Excel spreadsheet for access by the SDS macro.
Finally, there is a spreadsheet comprising the substances on the public ACOIN list with associated CAS numbers and which incorporates in many instances first aid instructions, exposure values, and other health related matters into the SDS and/or label when called up.
Click the link for an example of our 16 point SDS work
Label Preparation
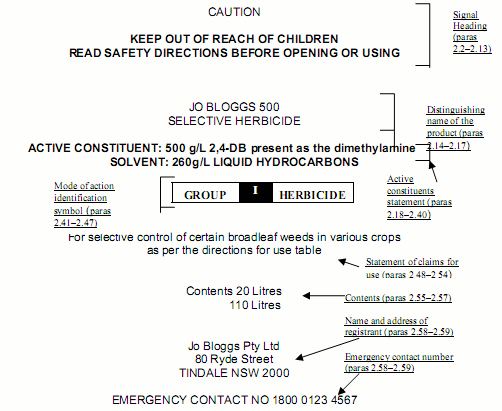
One of the services that goes hand-in-hand with Regulatory Affairs and SDS preparation is the determination of the text required by law in Australia. This can range from a few lines for products with no health hazards up to many pages for certain Agricultural chemicals.
The regulations can be quite bewildering to people who do not know their way around the regulations. The labels that can be seen on many supermarket and hardware shop shelves bear witness to the problems that the untrained person may have in trying to get it right.
We therefore offer a full service in this area, from the determination of necessary text and layout, through to proofreading of artwork prior to printing.
General Chemical Consulting
The Chemical Industry in Australia is much larger than most people believe. It supplies ingredients and formulated products to other industries such as the food, paper, plastics, agriculture, medical, veterinary and just about any other industry you can think of. While most larger companies have in-house skills and resources to investigate and report on various aspects of chemical related matters , many do not.
We offer our knowledge in a diverse number of matters such as health and safety, environmental interactions, compliance with regulations and so-on when those skills do not exist in-house. So, if you have a chemical related problem, please contact us. If we cannot help directly, we will probably know someone who can help you.